Up to 24 cash back On the photoelectron spectrum for magnesium given above draw the spectrum for aluminum. Up to 24 cash back Sketch the photoelectron spectrum of magnesium below then draw the spectrum for aluminum either on top of Mg or below it.
Up to 24 cash back 6.

. Label each peak with the appropriate shell and subshell. However say I am instructed to draw the PES graph for Silicon. Viewed 1k times 1 begingroup I understand the basic concepts of the photoelectron spectrum.
Explain the reasons for your diagram. Ask Question Asked 5 years 5 months ago. Consider the following PES spectrum a.
Label each peak with numbers and letters that indicate its associated shell and subshell. 1s2 2s2 2p6 3s2 3p6 - 3090 - 315 - 241 - 283 - 152 6. Boron aluminum hydride cluster anions B x Al y H z were generated in a pulsed arc cluster ionization source and identified by time-of-flight mass spectrometry.
In the analytical technique of photoelectron spectroscopy PES a sample is ionized using high-energy radiation and the kinetic energies of the ejected ele. A Give the electron configuration and identify the element. X-ray photoelectron spectroscopy XPS is a surface sensitive non-destructive technique used routinely to analyze the outermost 10 nm 30 atomic layers of natural and engineered materials.
Label each peak in the spectrum to show which subshell it represents ie 1s 2s etc On diagram above c. Indicate the number of electrons that are contained within each sublevel on your photoelectron spectrum for aluminum. Comparison between the well-resolved photoelectron spectra of Al n D 2 and Al reveals the nature of interactions between D 2 and Al n.
Suggest a reason for the huge jump in energy between peak A and peak B. These reference pages contain tips and techniques that are designed to help both the novice and advanced XPS user. On the spectrum sketch in the relative locations and correct peak heights for the spectrum of aluminum atomic number 13.
The binding energy of the K 3s electrons is greater than the Na 3s electrons 2 pts i The intensity of the peak of the K 3s electrons is higher than the Na 3s electrons 2 pts 15 050 4 35 25 Binding Energy MImol Identify the element whose photoelectron spectrum is given below. C Draw the photoelectron spectrum of the ion of this element superimposed on this diagram. Modified 3 years 11 months ago.
A Using the information from the PES spectra above write the electron configurations for i magnesium ii aluminum i State the anomaly in the first ionization energies shown on the table above. Photoelectron spectrophotometers use high-energy radiation UV or X-rays to eject electrons from an atom The photoelectron spectrophotometer inputs only one type of radiation with a specific energy Because electrons within an atom are in different energy levels different electrons can require different amounts of energy to eject. On the spectrum sketch in the relative locations and correct peak heights for the spectrum of aluminum atomic number 13.
XPS is routinely used to determine a the composition of material surfaces elemental identification the relative abundances of these components on. Suggest a reason for the huge jump in energy between peak A and. 5 Below is shown the PES spectrum of sulfur atomic number 16 a.
THE JOURNAL OF CHEMICAL PHYSICS 138 124303 2013 Photoelectron spectroscopy of the aluminum hydride anions. Up to 24 cash back 5. Using the plot write the electron configuration of the element and identify it.
Indicate the relative intensities and positions of all peaks. Given the photoelectron spectrum above which of the following best explains the relative positioning of the peaks on the horizontal axis. AlH 2 AlH 3 Al 2H 6 Al 3H 9 and Al 4H 12 Xinxing Zhang 1Haopeng Wang Evan Collins Alane Lim Gerd Ganteför2 Boggavarapu Kiran3 Hansgeorg Schnöckel4 Bryan Eichhorn5 and Kit Bowen1a 1Department of Chemistry Johns Hopkins.
Use the photoelectron spectrum below to answer the following questions. Draw a photoelectron spectrum for aluminum which includes all of the peaks but does not include exact ionization energies. O has more electron-electron repulsions in the 2p sublevel than Ti and C.
Consider the following PES spectrum 200 100 500 400 300 Binding Energy eV a. Draw a photoelectron spectmm for aluminum which includes all ofthe peaks but does not include exact ionization energies b. Spectra from aluminum cluster anions Aln 2 12.
15 Photoelectron Spectroscopy Electron Configuration 3. Indicate the number of electrons that are contained within each sublevel on your photoelectron spectrum for aluminum. Photoelectron spectroscopy is used to investigate aluminum dideuteride cluster anions Al nD 2 n3615 produced by laser vaporization of a pure Al target with a D 2-seeded helium carrier gas.
Draw a circle around the sulfur peak whose energy. A Look at the spectra of K and Na below. By relative location I mean correctly to the left or right of the same subshell peak in the sulfur spectrum.
Based on the information provided below draw a photoelectron spectrum for argon. Identify the element in the photoelectron spectrum shown below. 15 Photoelectron Spectroscopy Electron Configuration 3.
Label each peak with the appropriate shell and subshell. The calculated PES spectra obtained via shifting of the simulated electronic densities of states by the self-consistently determined. X-ray photoelectron spectroscopy XPS or ESCA curve fitting procedures reference materials and useful notes are listed here to provide a starting point for the consistent interpretation of XPS spectra.
B Draw the photoelectron spectrum of aluminum superimposed on this diagram. O has more valence electrons than Ti or C so more energy is required to remove them b. Electron affinity is the negative of the energy change that occurs when an electron is accepted by an atom in the gaseous state to form an anion.
Boron aluminum hydride clusters are studied through a synergetic combination of anion photoelectron spectroscopy and density functional theory based calculations. How to draw a photoelectron spectrum with ionization energies. The photoelectron Spectra charts for magnesium and aluminum are shown below.
Write the full electron configuration of sulfur b. Ii Using the information from the PES charts andor your electron. PHOTOELECTRON SPECTROSCOPY Background Information.
Using the plot write the electron configuration of the element and identify it. Label each peak With numbers and letters that indicate its associated shell and subshell c. For an in-class formative assessment you could try having students sketch spectra for specified elements.
After mass selection their.
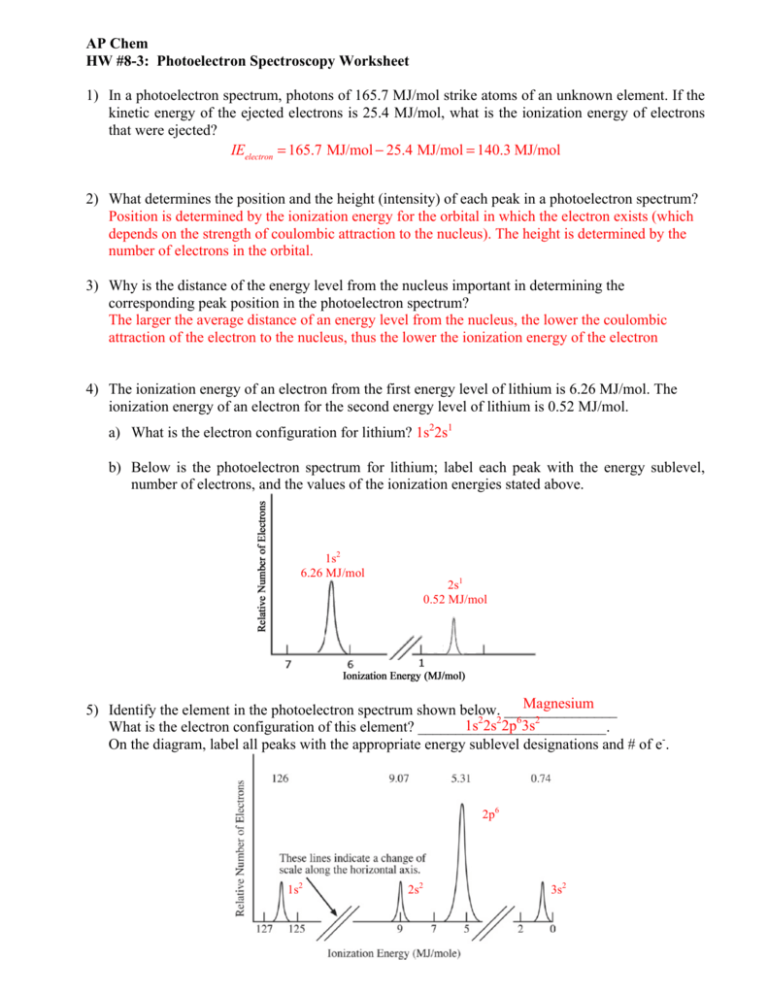
Hw 8 3 Photoelectron Spectroscopy Wks Key
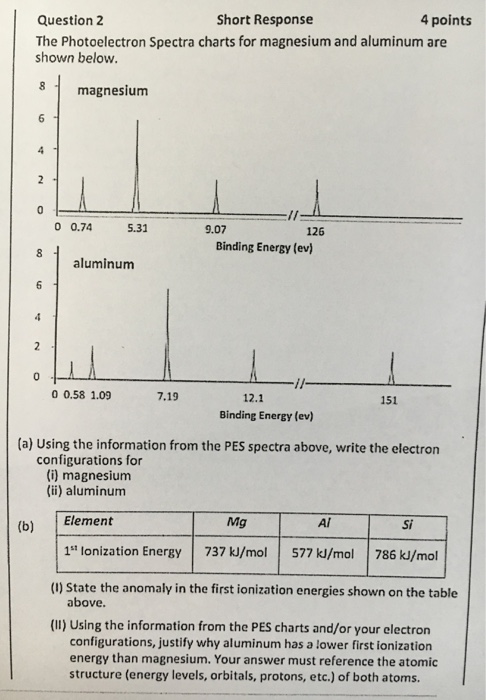
Solved The Photoelectron Spectra Charts For Magnesium And Chegg Com

Ap Chemistry Unit 2 2 Photoelectron Spectroscopy Ppt Download

Photoelectron Spectroscopy Article Khan Academy

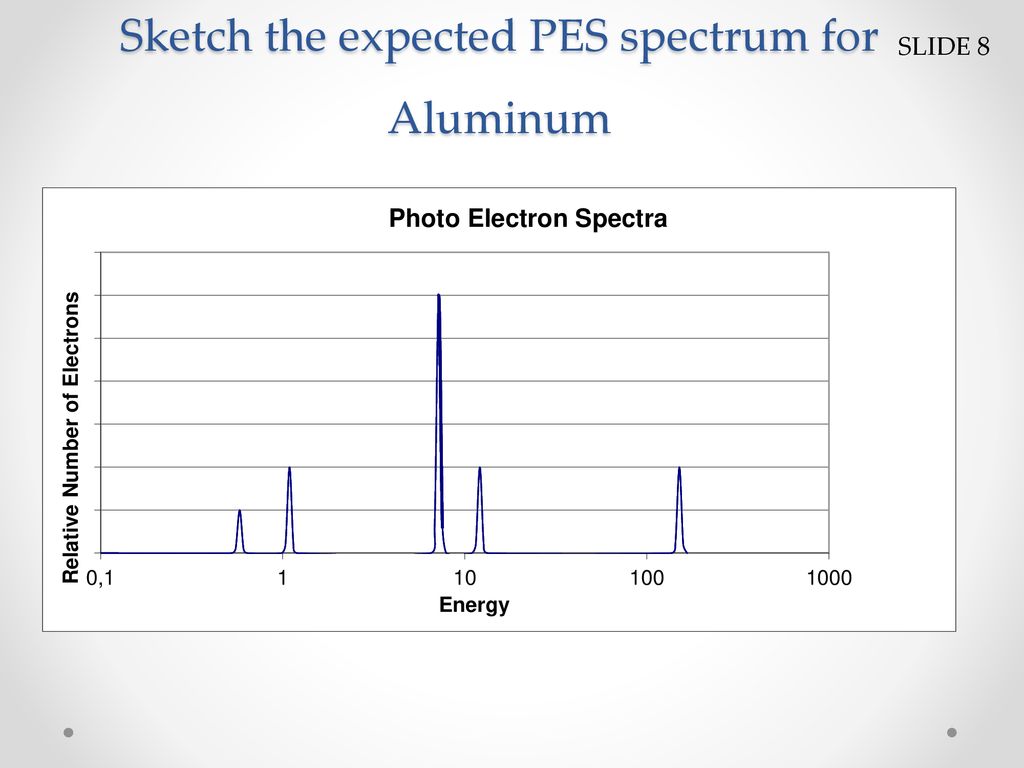
0 comments
Post a Comment